Physical Properties of Group 13 Elements
Physical Properties of Group 13 Elements: Overview
This topic covers concepts, such as, Physical Properties of Group 13 Elements, Melting Points of Group 13 Elements, Boiling Point of Gallium & Trend in Density of Group 13 Elements etc.
Important Questions on Physical Properties of Group 13 Elements
The incorrect statement from the following for borazine is:
Given below are two statements:
Statement I: Boron is extremely hard indicating its high lattice energy.
Statement II: Boron has highest melting and boiling point compared to its other group members.
In the light of the above statements, choose the most appropriate answer from the options given below
Which one of the following elements will remain as liquid inside pure boiling water?
Statement Boron is hard as it has high lattice energy.
Statement Boron has high melting and boiling points as compared to other group elements.
Which of the following element has the lowest boiling point ?
Which of the following element has highest density ?
The densities of boron family elements are more than
Write a short note on 'Boron is an Insulator'.
Why aluminum wire is generally used in power transmission lines and local power distribution lines.
Write a short note on Electrical conductivity of group 13 elements.
Order of standard reduction potential of group 13?
What is the boiling point of gallium?
Match List I with List II
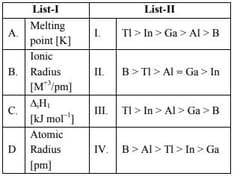
Choose the correct answer from the options given
below :
Given below are two statements :
Statement I : Gallium is used in the manufacturing of thermometers.
Statement II : A thermometer containing gallium is useful for measuring the freezing point (256 K) of brine solution.
In the light of the above statement, choose the correct answer from the options given below :
Match column I with column II
| Column I | Column II |
| P. Size | 1. |
| Q. Ionization enthalpy | 2. |
| R. Melting point | 3. |
| S. Ionic radius | 4. |
Assertion (A): Ga is used in thermometer
Reason (R): Melting point of Ga is low where as boiling point is high
Given below are two statements : one is labelled as Assertion and the other is labelled as Reason .
Assertion (A) : Melting point of Boron is unusually high in group elements.
Reason (R) : Solid Boron has very strong crystalline lattice.
In the light of the above statements, choose the most appropriate answer from the options given below ;
Assertion: Boron has high melting point (2437K)
Reason : Solid boron has strong crystalline lattice.
Boron has an exceptionally high melting point in the group elements, because–
The complex formed when is leached from bauxite using conc. solution is:
